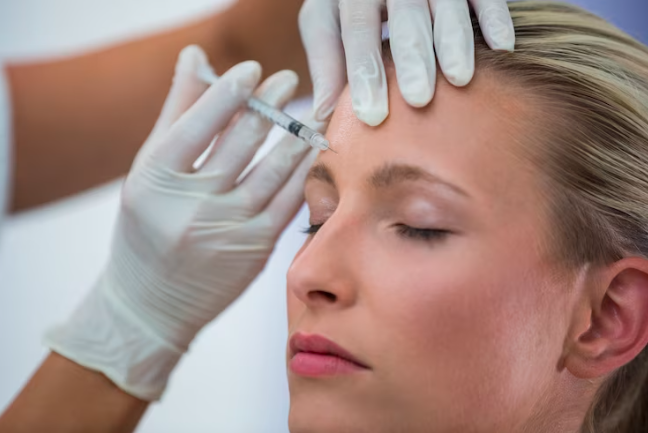
SINGAPORE: The Health Sciences Authority (HSA) is investigating a batch of dermal fillers to see if they are safe after a woman went blind following dermal filler treatment.
The incident, which occurred in July, is the first locally reported case of blindnesss resulting from dermal fillers, HSA said in response to queries from Channel News Asia (CNA) on September 12.
Dermal fillers are classified by HSA as Class D medical devices, which carry the highest risk.
The affected woman received AestheFill, a specific brand of dermal filler injected into the subcutaneous layer of skin for temporary improvement of facial wrinkles and folds.
AestheFill had been registered in Singapore since October 1, 2021.
Investigation focus: Batch-related defects and product safety
HSA is investigating whether there are any defects in the batch of AestheFill used that made it unsafe.
HSA said, “Should there be any product or batch-related issues, HSA will take the necessary actions such as to recall the affected product or require the company to rectify the issues.”
The AestheFill distributor, Parvus, promptly reported the incident to HSA on July 29, adhering to the rule that companies must report adverse events within ten days. CNA has asked for a comment from Parvus regarding the incident.
According to HSA, there hasn’t been a noticeable surge in adverse event reports related to aesthetic implants like dermal fillers.
Blood vessel occlusion, which can result in blindness, is a known risk associated with dermal fillers and is commonly mentioned as a potential adverse event in the Instructions For Use (IFU) provided to clinicians.
Known risks and common complications
The IFU for AestheFill stated the importance of avoiding injection into blood vessels, because it can lead to blood vessel occlusion. Other common complications reported following dermal filler treatments include swelling, redness, bumps in or under the skin, skin blanching, and temporary blurring of vision.
HSA emphasised that clinicians who administer dermal fillers like AestheFill are required to undergo training provided by the respective companies.
In the United States, the Food and Drug Administration (FDA) defines IFUs as FDA-approved patient labelling for drugs with complex or detailed patient-use instructions. It provides patients with step-by-step instructions for drug use, including how it should be prepared, administrated, handled, stored, and disposed.
Reactions from Reddit
With the ongoing investigation, discussions on social media platforms like Reddit have sparked questions and concerns.
One user commented, “What will happen to the doc who administer the filler?”
Another replied, “Nothing happen lorh, the doctor didn’t make the filler what”
A different perspective came from a user who wrote, “Fillers r v popular with aunties. Haha aesthetic docs gona make less $ now as this news goes around n they become scared”
A user said, “Everything in medicine comes with a certain risk. Take for instance, paracetamol a common OTC drug and even if you have taken it many times before can sometimes trigger Steven Johnson syndrome.”
With concern, he added, “It’s just very unfortunate that this routine procedure resulted in blindness, hopefully it’s not permanent.”
Some Reddit users also observed victim blaming and shared their thoughts about it.
One user expressed her dismay, saying, “Smlj? Why so much victim blaming in the comments? Some buttery smooth brain even say it’s God’s will? Imagine if the victim happens to be your relative, I wonder if you’ll still be victim blaming or rushing to pray and thank God for his will. Abhorrent behavior.”
She added stating, “What’s wrong with someone wanting to look pretty/young? Hopefully can reverse or be treated.”
Advice for consumers
HSA advises consumers to talk to their clinicians about the potential risks and suitability of dermal fillers before undergoing any such procedures. However, it’s important to remember that cosmetic surgeries and procedures do not fall under HSA’s jurisdiction.
CNA has reached out to the Singapore Medical Council, responsible for governing and regulating the conduct and ethics of registered medical practitioners, as well as the Society of Aesthetic Medicine and the Singapore Society of Ophthalmology for their take on the matter.
HSA is investigating the dermal fillers to ensure the safety and well-being of people getting dermal filler treatments in Singapore.
Read also:
Single-Pay vs Multi-Pay Critical Illness Plans – Singapore News