A team of researchers from Agency for Science, Technology and Research’s (A*STAR) Genome Institute of Singapore (GIS) has developed a new method to reveal ribonucleic acid (RNA) structures, and the way cells function.
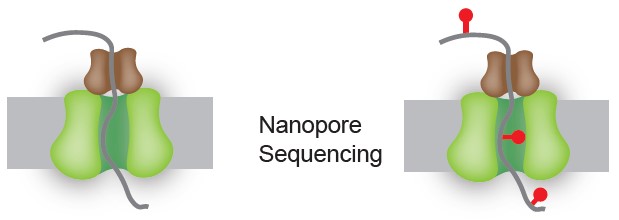
This is done by threading the RNA structures through protein pores and decoding the resulting signal. Using this novel method, the researchers have been able to shed light on the unique conformations that these molecules could adopt.
The researchers discovered that these molecules could have different structures despite being highly similar in sequence, and that these differences are associated with their unique function in the cell.
The human cell is extremely complex. One source of complexity comes from the fact that a single gene can be made into many different RNAs, which are then translated into different proteins. However, how these different RNAs are regulated remains to be understood.
One of the long-standing puzzles in biology is whether RNAs arising from the same gene could have different functions, and whether different structures have a major role to play in their diverse fate even if they share highly similar sequences.
In the past, it was not possible to discern individual structures in RNA populations with highly similar sequences, much less investigate the relationship between structure and function, because the technologies used for the readout of RNA structure can only generate short sequence reads.
The researchers overcame this problem by leveraging the recent development of nanopore sequencing and the long-read advantage that came with it. By running biochemically-modified RNA molecules through protein pores and decoding the resulting current with artificial intelligence, the researchers are able to obtain RNA structures in a fast and direct way to tell what RNA molecules and structures are present in a cell.
As a proof of concept, the authors performed their studies on human embryonic stem cells and showed that many RNAs arising from the same gene contain different structures that can impact the amount of proteins that are made inside the cell.
This technology has broad applications in diverse systems. Besides human cells, many pathogens (including RNA viruses such as dengue and Zika) also contain different transcripts with highly shared sequences. Application of this technology to studying RNA viruses in the future can elucidate how these pathogens function and serve as a basis for further research on how to target them.
Dr Wan Yue, Group Leader of Laboratory of RNA Genomics and Structure, and Associate Director of Epigenetic and Epitranscriptomic Systems at GIS, said, “Just like siblings are different from each other, RNAs made from the same gene can have very different shapes that govern their function.
“By using AI to convert current into structure in nanopore sequencing, we are able to study structure differences in RNA siblings to understand how each sibling work based on their shape.”
Prof Patrick Tan, Executive Director of GIS, added, “This method revolutionises our understanding of RNA-based gene regulation, and adds another tool in the tool box for mapping RNA structures in a high-throughput manner. Understanding RNA at the level of structure will elucidate a new universe of disease biomarkers and drug targets.”