SINGAPORE: Researchers from the Yong Loo Lin School of Medicine at the National University of Singapore (NUS Medicine) have pioneered a ground-breaking method to deliver therapeutic molecules directly to the brain by bypassing the blood-brain barrier (BBB).
Led by Dr Haosheng Shen, the study was published in the prestigious life sciences journal Cell and represents a significant step forward in treating neurological conditions.
Dr Shen, the lead researcher from the Synthetic Biology Translational Research Programme at NUS Medicine, collaborated with the NUS Synthetic Biology for Clinical and Technological Innovation (SynCTI) team. Their novel approach harnesses the power of a naturally occurring nasal bacterium, Lactobacillus plantarum (Lp), which has been genetically engineered to produce therapeutic compounds. These compounds are then released through a specific nose-to-brain pathway, offering a targeted method for brain drug delivery.
The BBB is a critical protective mechanism, safeguarding the brain from harmful substances, but it also complicates the delivery of medications for neurological disorders. Current drug delivery techniques face challenges due to inefficiencies and the invasive nature of many procedures.
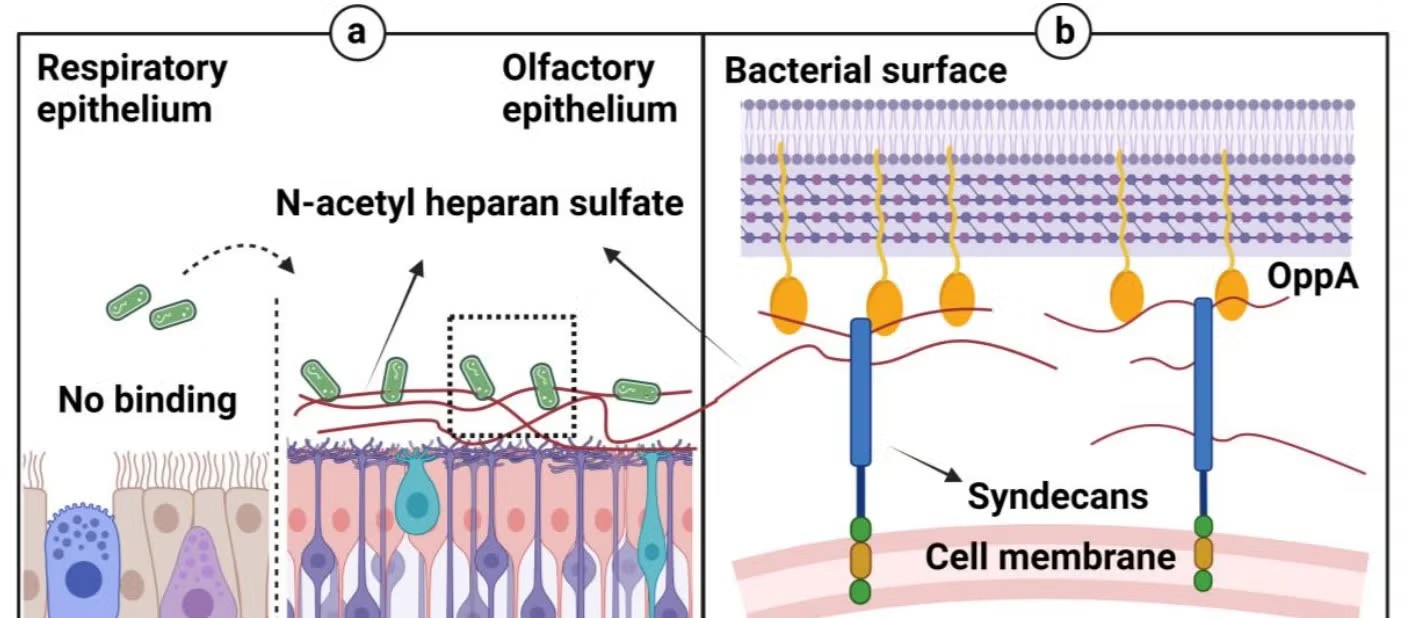
To address these issues, the research team identified a strain of Lp with a natural affinity for the olfactory mucosa, the tissue in the upper nasal cavity responsible for the sense of smell. This tissue provides a direct route to the central nervous system, making it an ideal site for intranasal drug delivery.
However, delivering drugs through the nasal cavity presents its own challenges, including the small surface area of the olfactory mucosa and the rapid clearance of substances by the body.
To overcome these obstacles, the team engineered the Lp strain to bind to N-acetyl heparan sulfate (NaHS), a sugar molecule found in the olfactory epithelium. This binding mechanism ensures a localized, sustained release of medication, maximizing bioavailability in the brain while minimizing systemic absorption.
In preclinical trials, the modified Lp strain was able to produce appetite-regulating hormones. The team demonstrated the potential of this engineered bacterium by showing that intranasal administration of the modified bacteria resulted in reduced appetite, improved glucose metabolism, lower body weight gain, and decreased fat accumulation. Remarkably, the medication reached the brain, highlighting the feasibility of this approach for targeting brain-related disorders.
“This study demonstrates the promise of engineered bacteria as precision delivery vehicles for brain-targeted therapies,” said Dr Shen. “Our findings could open the door to innovative treatments for neurological diseases, utilizing the often overlooked connection between the nasal microbiota and brain function.”
The team’s work is expected to have a far-reaching impact, offering new possibilities for the treatment of neurodegenerative diseases like Parkinson’s and Alzheimer’s. By bypassing the BBB, this novel technique could provide a more efficient method of delivering treatments for conditions that require peptides or proteins that typically struggle to cross the barrier.
Looking ahead, Dr Shen noted that the next phase of the research will focus on optimizing dosing regimens and conducting human clinical trials to evaluate the safety and efficacy of this approach. Additionally, the team plans to explore other therapeutic applications for this novel delivery system, particularly for neurodegenerative diseases.
Professor Matthew Chang, Director of the Synthetic Biology Translational Research Programme and SynCTI, emphasized the significance of the study: “Our research highlights the potential of nasal bacteria as non-invasive vehicles for brain drug delivery and underscores the importance of further exploring the relationship between the olfactory microbiome and brain health. This breakthrough could unlock new strategies for managing neurological disorders.”