SINGAPORE: Researchers at the Yong Loo Lin School of Medicine (NUS Medicine) have pioneered a breakthrough method to engineer yeast (Saccharomyces cerevisiae) capable of forming self-regulating microbial communities that adapt to environmental signals.
This innovation holds immense potential for advancing personalised healthcare, with applications ranging from targeted therapies to efficient biotech processes.
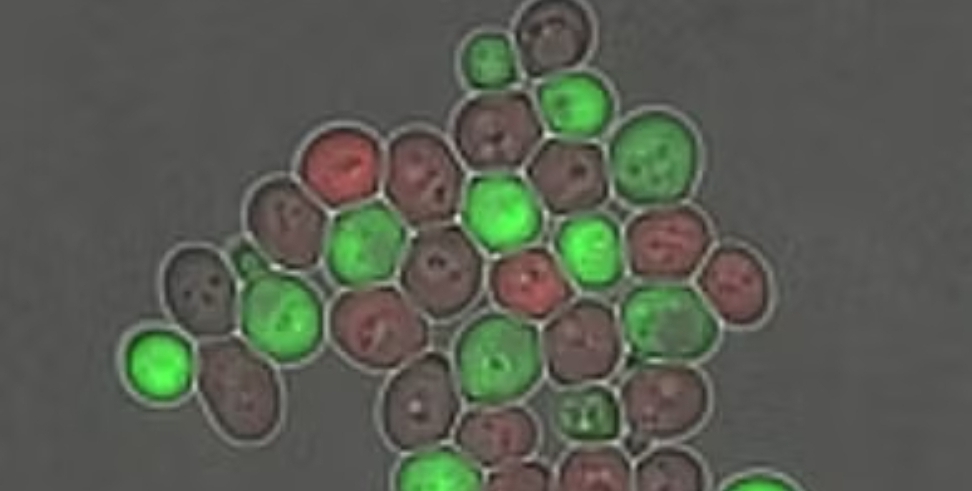
The NUS Medicine team reprogrammed yeast cells to switch between specialised types, enabling them to form cooperative ecosystems that can autonomously adjust their population balance.
This development marks a significant leap from traditional microbial biotechnology, constrained by its reliance on single-cell organisms incapable of executing complex, coordinated tasks.
The engineered yeast cells operate similarly to natural microbial ecosystems. By splitting into two specialised types, they work synergistically to share tasks, self-regulate their structure, and respond dynamically to external stimuli.
This capability is particularly promising for precision medicine, where therapies must adapt to changing patient conditions in real-time.
“These artificially engineered smart yeast cells could revolutionise how microbial communities are controlled for health purposes,” said Associate Professor Matthew Chang, Director of the Synthetic Biology Translational Research Programme at NUS Medicine and NUS Synthetic Biology for Clinical and Technological Innovation.
“By dividing tasks and autonomously adjusting their balance, these yeast systems reduce the stress on individual cells, improving their efficiency and reliability.”
The smart yeast functions as microscopic factories capable of producing therapeutic compounds or breaking down complex substances into simpler forms.
Crucially, the yeast reacts to disease markers—molecules that accumulate in the body during illness—allowing it to produce precise amounts of therapeutic compounds as needed.
This smart regulation ensures minimal waste, increased efficiency, and reduced side effects, making therapies safer and more effective.
A/Prof Chang highlighted the impact of this approach on healthcare: “In the gut, for example, these yeast cells can autonomously balance their activity in response to health signals, such as disease markers.
This allows for flexible and precise therapeutic production without manual intervention, improving treatment outcomes.”
The research team is currently optimising the engineered yeast’s adaptability to various disease markers. Their next step is to evaluate the efficacy of these self-regulating microbial communities in producing health-enhancing molecules for the treatment of specific diseases.
Beyond personalised healthcare, this innovation also offers promising applications in the biotech sector.
The engineered yeast systems improve the sustainability, scalability, and precision of biotechnological processes, paving the way for more efficient production of therapeutic compounds and other valuable substances.
This cutting-edge research represents a transformative step toward smarter, more adaptive treatments and technologies, setting the stage for advancements in medicine and industrial biotechnology.